Recording and Q&A of DCAA family meeting
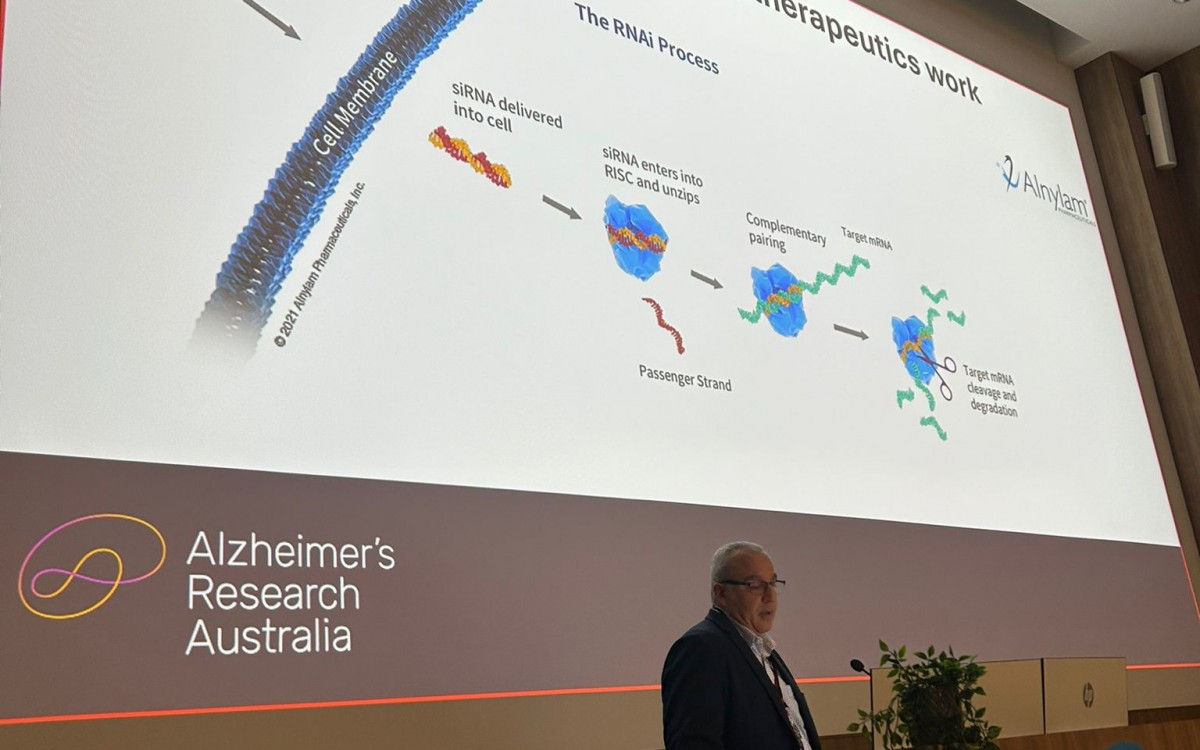
The first drug trial in DCAA is here. Alnylam Pharmaceuticals is developing Mivelsiran, a RNAi therapy that targets amyloid production. A phase 2 study, focused on safety and efficacy, is ongoing since 2024. 152 Patients with sporadic CAA will be enrolled in the study, and up to 48 DCAA genecarriers from the age of 30.
Together with Alzheimer's Research Australia, the DCAA patient association organised a DCAA family meeting in Perth, to inform you about the drug trial and participation in the study. Another goal of the DCAA family meeting was to facilitate peer support, inform about the work of the DCAA patient association in the Netherlands, and to answer questions about ongoing research and healthcare.
During the meeting the ARA team went into the specifics of the drug trial. What does the drug do? What does a phase 2 mean? Who can participate and why? What is being asked of participants? Out of the perspective of the lived experience, Sanne presented a timeline of how we got here, and what to consider when someone is considering enrolling in the trial. Maike told the attendees about why she participates in DCAA research as a genecarrier, what she experiences during study visits and how she feels during and after those days.
The recording of the meeting and the Q&A session is now available here. An overview of the Q&A can be found here.
If you have any questions based on the recording, please do not hesitate to contact Sanne at svanrijn@hchwa-d.nl. If you want to connect with the research team directly, contact Samantha Gardener at s.gardener@ecu.edu.au.
Big thanks again to the Alzheimer's Research Australia team for the time and effort put into DCAA research and DCAA family meetings. Also, thanks to Alnylam Pharmaceuticals for sponsoring the meeting*.
*Alnylam Pharmaceuticals did not sponsor for recruitment purposes and only to help support Australian DCAA family members.